Introduction
Clinical trials are a cornerstone of medical advancement, providing essential data on the safety and efficacy of new treatments. Traditionally, these trials have been researcher-centric, focusing primarily on the needs and protocols of the scientific community. However, there is a growing shift towards patient-centric approaches, which place the needs, convenience, and experiences of patients at the forefront. This article explores current trends and emerging strategies in patient-centric clinical trials, emphasizing the importance of this paradigm shift for improving patient outcomes and trial efficiency.
What is Patient-Centricity in Clinical Trials?
Patient-centricity refers to designing and conducting clinical trials with a primary focus on the patients’ needs, preferences, and overall experience. This approach aims to make participation in clinical trials more accessible, convenient, and less burdensome for patients. By involving patients as active partners rather than passive subjects, patient-centric trials can improve recruitment, retention, and data quality.
Current Trends in Patient-Centric Clinical Trials
1. Use of Telehealth and Remote Technology
Telehealth has revolutionized clinical trials by enabling remote participation. Patients can attend virtual visits, reducing the need for travel and making trials more accessible, especially for those in remote or underserved areas. Remote technology such as smartphones, wearable devices, and computers allows for continuous monitoring of patient health data, providing researchers with real-time insights.
For instance, Medable, a digital health platform, has been instrumental in transforming clinical trials with its suite of telehealth and remote monitoring tools. Medable’s platform supports decentralized trials by integrating electronic consent (eConsent), telemedicine visits, and remote patient monitoring. Their technology enables continuous data collection through wearable devices, enhancing the accuracy and comprehensiveness of clinical trial data.
2. Decentralized Clinical Trial Models
The decentralized model leverages digital tools to conduct trials outside traditional clinical settings. Patients can participate from home, reducing logistical challenges and enhancing convenience. This model has been particularly effective during the COVID-19 pandemic, demonstrating its potential for broader application.
Labcorp Drug Development has embraced decentralized clinical trials by offering home healthcare visits and leveraging digital health technologies. Their approach includes using mobile nurses for in-home visits and remote sample collection, which minimizes patient burden and increases participation rates. Labcorp’s decentralized model has shown significant promise in enhancing patient recruitment and retention.
3. Enhanced Patient Recruitment and Retention Strategies
Recruitment and retention are critical challenges in clinical trials. Patient recruitment companies and innovative strategies such as targeted social media campaigns, patient advocacy organizations, and community engagement are being employed to reach potential participants. Retention efforts focus on minimizing the drop-out rate through regular follow-ups, patient feedback forms, and ensuring a positive trial experience.
IQVIA, a global provider of advanced analytics, technology solutions, and clinical research services, utilizes artificial intelligence (AI) and machine learning to identify and engage potential trial participants. Their technology analyzes electronic health records (EHRs) and other data sources to match patients with appropriate trials, streamlining the recruitment process. IQVIA’s AI-driven approach not only improves recruitment efficiency but also enhances patient retention by ensuring that participants are well-suited to the trials they join.
Emerging Strategies in Patient-Centric Clinical Trials
1. Personalized Patient Support
Providing personalized support to patients throughout the trial process is crucial. This includes offering at-home visits, childcare, transportation assistance, and reimbursement for travel costs. Such measures address practical barriers and improve patient compliance and satisfaction.
Parexel, a leading biopharmaceutical services company, has implemented personalized patient support programs that include telehealth services, home healthcare visits, and dedicated patient coordinators. Parexel’s initiatives ensure that patients receive the support they need throughout the trial, from initial enrollment to completion. Their personalized approach has significantly improved patient engagement and adherence, resulting in higher-quality data and more efficient trials.
2. Improved Informed Consent Process
The informed consent process is being enhanced to ensure patients fully understand the trial they are participating in. This includes using plain language, providing translations, and implementing eConsent solutions that allow patients to review and sign documents electronically.
Signant Health, a global leader in clinical trial technology, offers eConsent solutions that transform the traditional consent process. Their platform provides interactive multimedia content to explain trial procedures, risks, and benefits in a clear and engaging manner. Signant Health’s eConsent technology supports multiple languages and is accessible on various devices, ensuring that all patients can fully understand and comfortably participate in the consent process.
3. Incorporating Patient Feedback
Actively seeking and incorporating patient input into trial design and execution is essential for a patient-centric approach. Patient advisory boards and direct feedback mechanisms help ensure that trials are designed with patient needs in mind, leading to more patient-friendly protocols.
Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson, has established patient advisory boards to gather insights directly from patients and caregivers. These boards provide valuable feedback on trial design, patient communication, and overall trial experience. Janssen’s commitment to incorporating patient perspectives has resulted in more patient-friendly protocols and improved trial outcomes.
Challenges and Opportunities
Challenges
Despite the advantages, there are challenges to implementing patient-centric approaches. These include:
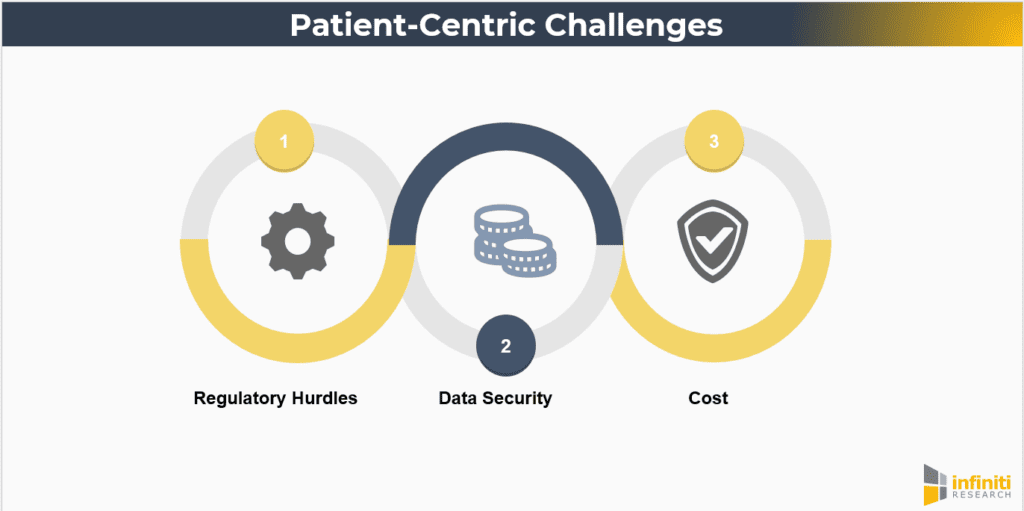
- Regulatory Hurdles: Ensuring compliance with regulatory standards while adopting new technologies and methodologies. Regulatory agencies are increasingly recognizing the value of patient-centric approaches, but navigating the evolving regulatory landscape requires careful planning and collaboration.
- Data Security: Protecting patient data privacy in a digital, decentralized environment. As trials become more reliant on digital tools, ensuring robust cybersecurity measures and maintaining patient confidentiality are paramount.
- Cost: The initial investment required for technology and infrastructure upgrades. While patient-centric approaches can lead to cost savings in the long term, the upfront costs associated with new technology, training, and process redesign can be significant.
Opportunities
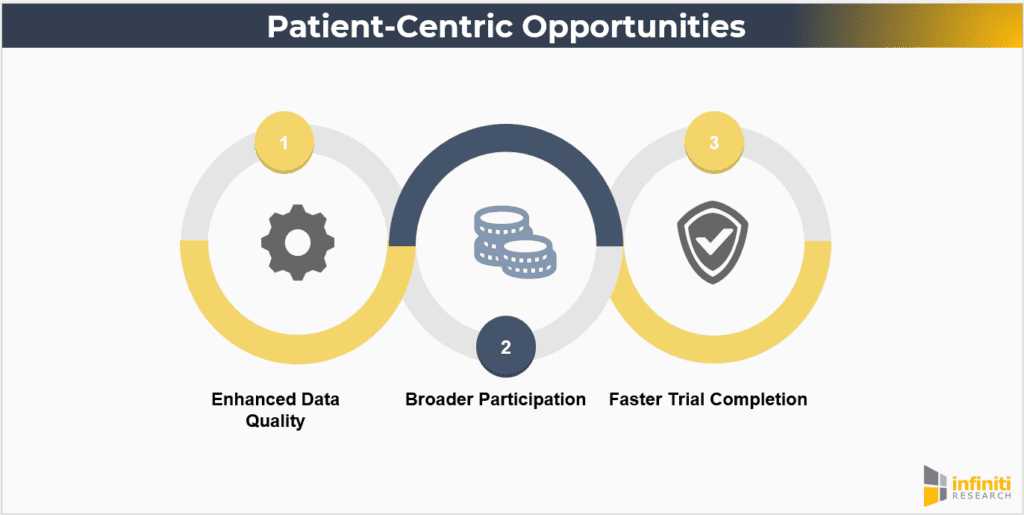
- Enhanced Data Quality: Patient-centric trials can lead to more comprehensive and higher-quality data due to better patient engagement and compliance. When patients are more engaged and feel that their needs are being addressed, they are more likely to adhere to trial protocols and provide accurate data.
- Broader Participation: By reducing barriers, these trials can include a more diverse patient population, improving the generalizability of findings. Inclusivity is critical for ensuring that trial results are applicable to the broader population and for addressing health disparities.
- Faster Trial Completion: Improved recruitment and retention rates can expedite the trial process, bringing new treatments to market more quickly. Streamlined processes, reduced drop-out rates, and efficient data collection can significantly shorten trial timelines.
Conclusion
Patient-centric approaches in clinical trials represent a significant shift towards more inclusive, efficient, and patient-friendly research. By prioritizing the needs and experiences of patients, these approaches can improve trial outcomes, enhance data quality, and accelerate the development of new treatments. As the industry continues to evolve, embracing patient-centric strategies will be crucial for the future of clinical research.


